

In addition, human plasma treated with rivaroxaban, apixaban, heparin, and enoxaparin did not have their anti-FXa effects reversed when tested with idarucizumab. There was a weak effect on melagatran, which van Ryn noted has some similarities to dabigatran.
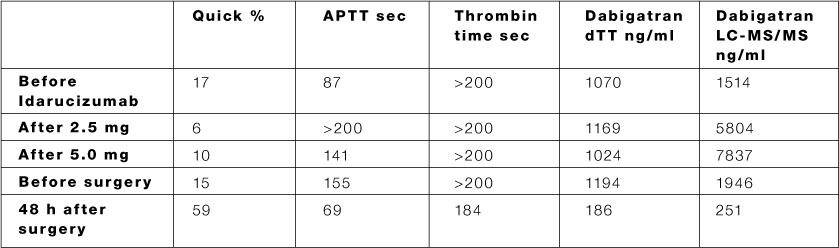
Idarucizumab did not reverse the effects of direct thrombin inhibitors (DTIs) hirudim, bivalirudin, or argatroban. Van Ryn then outlined another series of tests that analyzed whether idarucizumab would reverse the anticoagulant effects of several other agents. Later in the session, however, both van Ryn and moderator Steven Lentz, MD, of the University of Iowa, cautioned that this was not an invitation to purposely use PCCs along with idarucizumab if a patient’s dabigatran status is known in an emergency situation. 2Īccording to van Ryn, as well as an accompanying abstract, idarucizumab reversed anticoagulant activity of dabigatran to baseline this reversal was not affected by presence of PCCs, aPCC or rFVIIa. Researchers measured the levels of anticoagulant activity. Van Ryn Dabigatran (0-500 ng-mL) was added to human plasma that contained these coagulants, with or without idarucizumab (3.0 mg/mL). Van Ryn then reviewed results of a series of tests performed with idarucizumab and dabigatran in combination with coagulation factors 3- or 4-factor prothrombin complex (PCCs) activated PCC (aPCC) or recombinant Factor Villa (rFVlla). “Dabigatran is almost completely enclosed,” she explained. Joanne van Ryn, PhD, a scientist with Boehringer Ingelheim, first outlined the nature of the idarucizumab molecule, which binds to dabigatran in a 1-to-1 manner. In particular, the first scenario might occur in an emergency. 1Īccording to a presentation Monday at the American Heart Association Scientific Sessions, being held in Orlando, Florida, it remained to be seen how idarucizumab would work if a patient had already been given other coagulation factors, or how it might affect other anticoagulants on the market. Both Praxbind and Pradaxa are made by Boehringer Ingelheim. According to FDA, the accelerated approval was granted based on an unmet need. Last month, the FDA approved idarucizumab (marketed as Praxbind) as an agent that specifically reverses the anticoagulant dabigatran (approved in 2010 as Pradaxa) to prevent stroke and systemic blood clots in patients with atrial fibrillation and to treat deep vein thrombosis and pulmonary embolism.


 0 kommentar(er)
0 kommentar(er)
